Back in 12th grade, my chemistry book had this intriguing chapter named “Chemistry in Everyday Life”. At the time, it seemed like just another chapter amidst the jumble of formulas and reactions.
To be honest, I wasn’t too thrilled about chemistry. I was more inclined toward computers, constantly questioning the relevance of delving into chemical equations that appeared distant from my future. Like, what’s the point of learning this Wurtz Reaction? even though I know that I’m going to be a software engineer. How’s this going to help me in my life?
During my 12th final exams, I kind of took a shortcut and skipped over the organic chemistry chapters. Yep, all of them. It was a hasty attempt to save time or perhaps avoid the complexity that they seemed to hold.
All I did was that just a few hours before the exam, I decided to quickly breeze through all of the back questions/exercises associated with these chapters, for this also of course, I just found the solutions online and quickly read through all of them.
To my astonishment, when the results came out, it was a complete plot twist. I scored way better in chemistry than I ever imagined. It was a surprising turn, especially since my favorite subject, Physics, didn’t turn out as well as I’d hoped. I anticipated scoring around 85-90 marks at best in chemistry, but ended up scoring around 99, which was the highest amongst all in my school. (I still don’t know, how)
It got me thinking. Life has this way of surprising us, doesn’t it? That’s when I began to reconsider my notions about chemistry. Maybe it’s not that bad, huh?
But after that success, I thought I was done with it.
Until…
I encountered a stubborn, messy tawa in my new place in Bangalore!

No matter what I tried, those oil stains refused to budge! I attempted everything at my disposal. I experimented with various dishwashing liquids, hoping they would do the trick 🤞🏻.
I even tried pouring boiling water and scrubbing with all my might, but the stains seemed to have made themselves at home on the tawa’s surface. I resorted to scraping at it, hoping that the grime would yield, to no effect 😞
and this was the state after just 2 months of usage! I was this 🤌🏻 close to dispose this one off and buying a new tawa. However, Curiosity led me to investigate the composition of this stubborn black residue.
What the heck is this? Is this oil? Are these carbon stains from those overcooked chappatis? Turns out, it was just plain carbon. Some online sources suggested a simple concoction of vinegar (acetic acid) and baking soda. And guess what? It worked like a charm!
It was like, I had found my perfect vial of Felix Felicis (more commonly known as ‘liquid luck’)! 🙂

Although, I am a Harry Potter fan, I’m still a big believer of science :) So, I was eager to uncover the ‘magic’ behind this cleaning potion.
The chemical reaction between baking soda (sodium bicarbonate, NaHCO3) and acetic acid (vinegar, CH3COOH) that worked its wonders on the tawa’s grime is as follows:
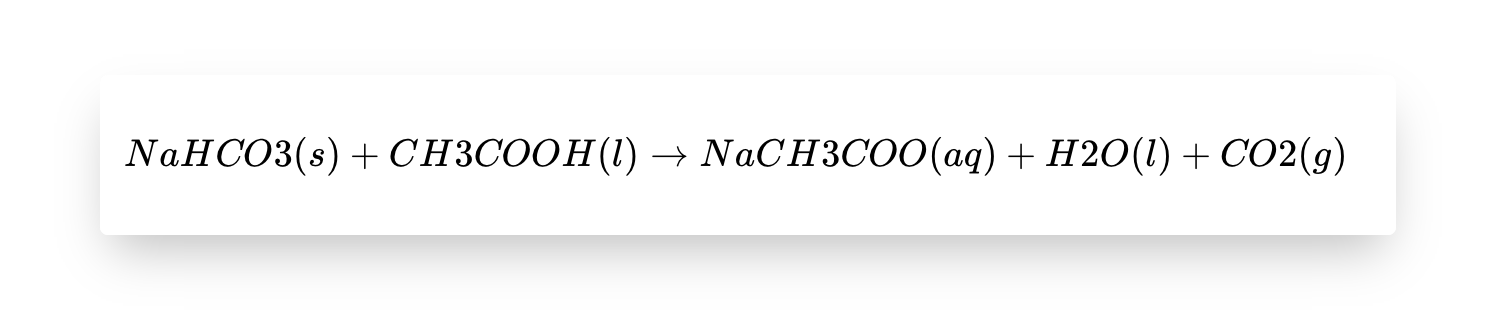
The reaction produces sodium acetate (NaCH3COO, dissolved in water), water (H2O), and carbon dioxide gas (CO2). The carbon dioxide gas is what creates the fizzing effect when the two substances combine.
But, this still doesn’t explain how it was able to clean that grime? 🤔
I spent hours on figuring this out. After enough thinking, reading up about this on the internet, asking chat gpt and validating my findings by discussing this with some of my JEE friends, I finally found the answer! 💡
Any guesses?
Hmmm. So…
The cleaning magic happened because of the carbon dioxide gas released during the reaction! As the baking soda and vinegar combine, the resulting carbon dioxide forms numerous tiny bubbles. When these bubbles come into contact with the soot and grime on the tawa, they help dislodge and lift it off the surface.
The bubbling action acts as a mechanical force that agitates the surface, loosening the dirt particles. This mechanical agitation helps break the bond between the grime and the tawa, making it easier to clean. The pressure from the bubbles lifts the stubborn stains, letting them easily wash away.
In addition to the mechanical action, the chemical reaction might also contribute in breaking down some of the organic components present in the soot, further assisting in the cleaning process.
But, The fizzing or bubbling effect is the key! It’s this ‘invisible force’ created by the chemical reaction that makes this cleaning concoction work wonders on stubborn grime and grease, leaving your utensils sparkling clean.
Remarkable, isn’t it?
Who would’ve thought back in 12th grade that a dash of chemistry would later step in, unexpectedly assisting me in various situations? 🙂
Don’t want to get too philosophical, but doesn’t it just go to show that everything happens for a reason, and every single experience, no matter how unexpected, has a way of shaping our future paths? :)
P.S. As an added bonus, here’s a short clip of me performing the above experiments :)